
With the electric vehicle market driving a surge in demand for lithium-ion batteries, research is intensifying to develop new electrolytes that not only ensure battery performance, lifetime, and safety, but also enable high-energy anode materials, such as silicon, and cathode materials, such as NMC 811. High purity electrolyte components are crucial to mitigate both side reactions and premature degradation of the battery. Arkema’s new electrolyte additive, LiTDI, not only increases battery lifetime and fast charging performance but also addresses these issues of purity and stability with high capacity battery materials, necessary for EV batteries.
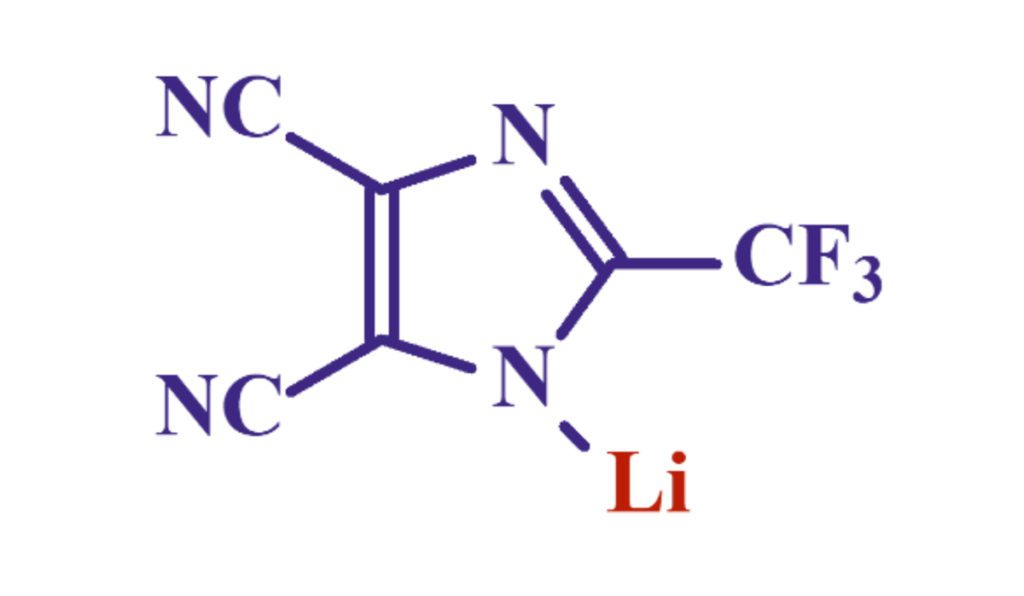
LiTDI, Lithium 4,5-dicyano-2-(trifluoromethyl)imidazole, was first discovered at Warsaw University of Technology (WUT). Its synthesis and purification have been the scope of collaboration between WUT, CRNS (French National Center for Scientific Research) and University of Amiens, France. As a member of Michel Armand’s team at the University of Amiens, Dr. Gregory Schmidt started to investigate the benefits of this lithium salt in lithium-ion batteries. Dr. Schmidt’s research focused on both synthesis and electrolyte formulation, demonstrating that this lithium salt would add great value to electrolytes, if used as an additive. After two years of application research and development in Amiens, he then returned to Arkema to incorporate this molecule into Arkema’s strong platform of battery and renewable energy solutions.
————————————–
Lithium TDI combines multiple benefits. Its molecular structure is specifically tailored to bring a great electrochemical stability as well as a good ion dissociation. First, the imidazole ring facilitates the negative charge delocalization via resonance effect. Second, the two nitrile groups have an optimized electronegativity/weight ratio that further helps the positive charge dissociate. Lastly, the CF3 group attached to the imidazole ring brings the electrochemical stability. Cyclic voltammetry studies show no reactivity of the molecule before 4.6 ~ 4.7V. Additionally, the molecule is extremely thermally stable, degrading only at temperatures above 250°C, as illustrated by DSC studies. As a result, this makes the molecule a great choice for long-lasting lithium-ion batteries operating at high voltage and high temperature.
Scavenging moisture impurities to increase electrolyte stability
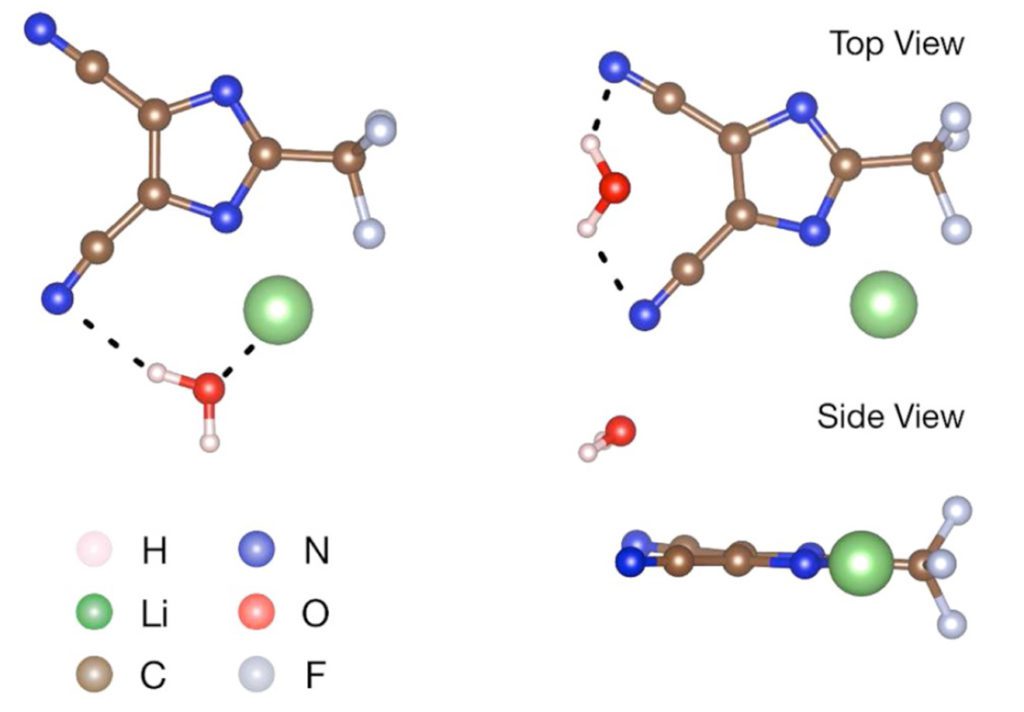
In addition to its inherent stability, LiTDI acts as a great moisture scavenger within the electrolyte: the lithium-ion and carbonitrile groups interact with water molecules to trap them via hydrogen bonding as emphasized by Xu’s team in a paper published in 2017 (Xu and all, Chem. Mater.2017.29.5.2254-2263). This interaction with water molecules would efficiently suppress the hydrolysis of LiPF5, a decomposition product of LiPF6 on the anode which is a strong Lewis acid, known as a primary source of electrolyte solvent degradations. Moreover, the carbonitrile groups interact with HF molecules, also resulting from LiPF6 degradation, to mitigate further parasitic reactions on the cathode side. By reducing the impact of impurities on the different electrolyte components, the addition of just 1% of LiTDI enhances the stability of the electrolyte, and thus battery lifetime.
Another significant impact of LiTDI on battery performance is its contribution to forming a passivation layer on the aluminum current collector. While researchers are trying to find alternatives to LiPF6 for high voltage applications, corrosion on the cathode current collector appears to be an issue, thus increasing the internal resistivity and reducing the cathode capacity. This stable aluminum protection formed by LiTDI (Paillet and all, J. Power Sources, 2015, 299, 309) increases the life of batteries containing new electrolytes based on alternative salts such as LiFSI, supporting the adoption of high voltage electrodes, such as NMC 622.
Decreased resistivity for fast charging
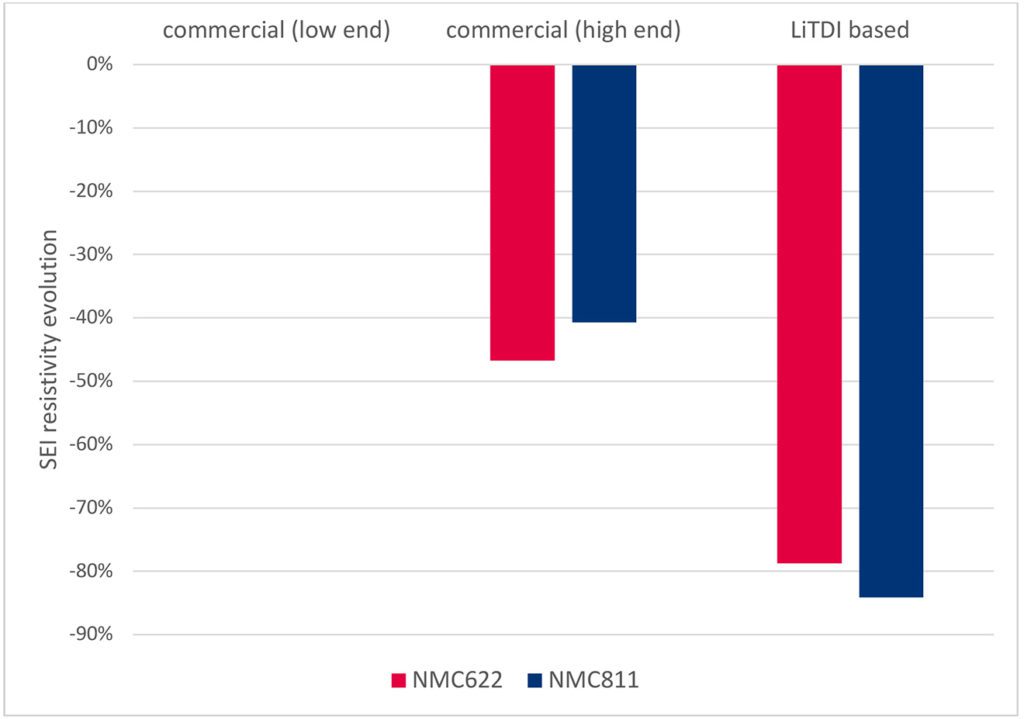
Lastly, the list of LiTDI properties would not be exhaustive without highlighting the important role it plays in the formation and stabilization of the Solid Electrolyte Interface (SEI), protecting the anode from degradation reactions with the organic solvents. In combination with traditional SEI additives, like FEC or VC, LiTDI helps grow the LiF mineral phase by defluorination of the CF3 group while fostering the formation of the polymeric phase (Shkrob and all, J. Phys. Chem. C 2016, 120, 50, 28463-28471). The resulting SEI is thinner, more cross-linked, and more robust, which translates into lower resistivity and a reduction in the initial capacity loss. Such phenomena have not only been observed on graphitic anodes but also on silicon-based anodes, where the role of SEI additives is even more crucial for battery life and battery internal resistance.
NMC111 cycling at 45C
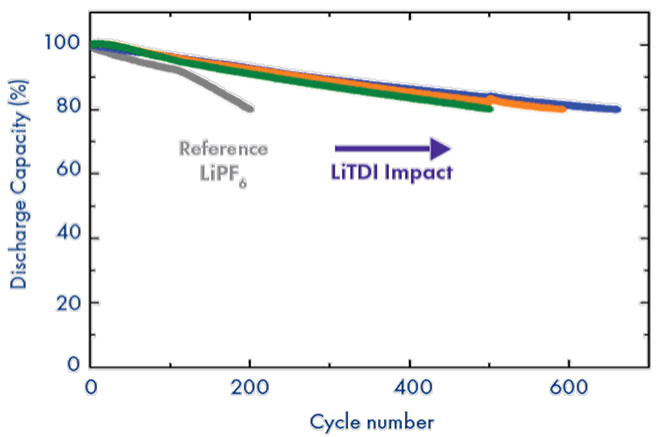
As a consequence of all the electrochemical benefits illustrated above, cells containing LiTDI as an additive, in synergy with traditional SEI additives, exhibit significant improvements in fast charge and discharge performance thanks to the reduced cell impedance. Additionally, the electrolyte purity and stability facilitated by this salt enables high-temperature cycling (>45°C) of traditional electrolytes. Finally, LiTDI is a great electrolyte additive to significantly extend battery life not only for graphitic anodes but also for silicon-based anodes.
source https://chargedevs.com/newswire/litdi-an-electrolyte-additive-for-extended-battery-lifetime-and-fast-charging/
No comments:
Post a Comment